Shelagh Fogarty 1pm - 4pm
30 April 2020, 21:22
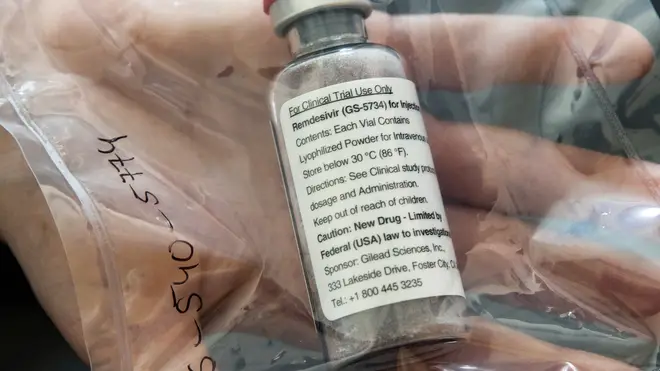
Trials for an Ebola drug which could treat coronavirus as "a really promising first step" towards overcoming the disease by leading medical experts.
Preliminary clinical trials of remdesivir suggest it could speed up recovery time for Covid-19 patients, with more research needed to determine whether it can reduce deaths.
Speaking at Thursday's coronavirus press conference, chief scientific adviser Sir Patrick Vallance said the drug "absolutely does hit a particular part of the virus", but warned it is not a "magic bullet."
A study in the US showed it aids recovery time by four days and trials are continuing, including one in the UK, he confirmed.
Sir Vallance said: "It's definitely not a magic bullet to cure this, but it does show that drugs are going to be possible to have an effect on this virus, and therefore there will be more that come along, and ultimately it may need to be a combination of drugs that come together to make this really effective."

Boris Johnson: we've passed the coronavirus peak
Chief medical adviser Professor Chris Whitty agreed the preliminary results have been "encouraging", but warned against "over-interpreting" the drug's potential until a peer-reviewed study is published.
He added a combination of drugs is likely to be needed to overcome the virus.
"What tends to happen is, through incremental steps, you move forward steadily improving, sometimes using combinations of drugs, each of which has a small effect, but when you put them together the added effect is very considerable," he said.
Prof Whitty also thanked those participating in drugs trials, adding the UK is recruiting volunteers "at an absolutely extraordinary rate."
More than 1,000 patients have been recruited across the world, including more than 45 from the UK, for the Adaptive Covid-19 Treatment Trial which began at the start of April.
Listen & subscribe: Global Player | Apple Podcasts | Google Podcasts | Spotify
During the trial, which involved more than 70 hospitals across the globe, patients were given the antiviral drug every day for 10 days while they remained in hospital.
Scientists involved in the study defined recovery as a patient being well enough to come off oxygen, being discharged from hospital or even returning to normal activity levels.
Professor Mahesh Parmar, director of the MRC Clinical Trials Unit at UCL, had previously said scientists will continue to gather data while the early results are reviewed by regulators.
"Before this drug can be made more widely available, a number of things need to happen: the data and results need to be reviewed by the regulators to assess whether the drug can be licensed and then they need assessment by the relevant health authorities in various countries," he said.
"While this is happening, we will obtain more and longer term data from this trial, and other ones, on whether the drug also prevents deaths from Covid-19."
There have been no significant side effects noted in patients participating in the trials.
Remdesivir, which was originally developed to treat Ebola, is one of a handful of experimental drugs undergoing clinical trials worldwide to treat coronavirus.