Nick Ferrari 7am - 10am
6 April 2021, 18:59 | Updated: 6 April 2021, 21:34
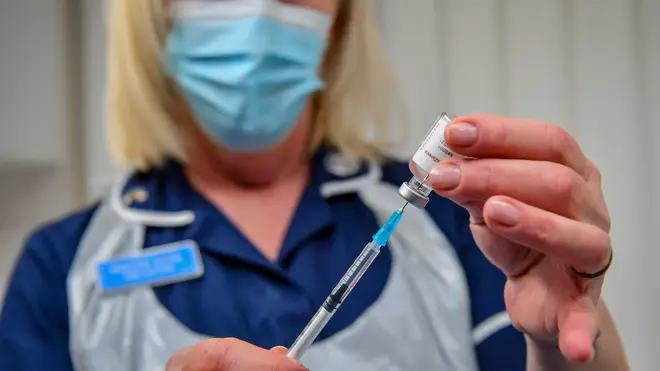
The Oxford/AstraZeneca vaccine trial on children has been paused while a possible link to blood clots is investigated.
The University of Oxford said it had paused giving doses of the vaccine in the study while it waits for further information about the rare problem in adults who received it.
The Medicines and Healthcare products Regulatory Agency (MHRA) is investigating the potential association between the jab and a form of blood clot.
A spokesperson from the university said in a statement: "Whilst there are no safety concerns in the paediatric clinical trial, we await additional information from the MHRA on its review of rare cases of thrombosis/thrombocytopaenia that have been reported in adults, before giving any further vaccinations in the trial.
"Parents and children should continue to attend all scheduled visits and can contact the trial sites if they have any questions."
Read more: Number of first vaccine doses in UK drops to lowest daily level since early January
Read more: US trials show Oxford vaccine safe and effective

Regulators in Europe and the World Health Organisation (WHO) are determining data about the jab's potential links to blood clots.
The WHO and the European Medicines Agency (EMA) said they will publish their findings later this week.
Earlier on Tuesday, Boris Johnson defended the vaccine at the AstraZeneca plant in Macclesfield, Cheshire.
Read more: France and Germany suspend use of Oxford/AstraZeneca Covid-19 jab
Read more: Seven die of blood clots in UK after AstraZeneca jab but no evidence of link
He said: "The best thing people should do is look at what the MHRA say, our independent regulator - that's why we have them, that's why they are independent.
"Their advice to people is to keep going out there, get your jab, get your second jab."
He added: "The best thing of all is to vaccinate our population, get everybody out getting the jab, that's the key thing and that's what I would advocate, number one."