
Shelagh Fogarty 1pm - 4pm
3 February 2021, 13:59
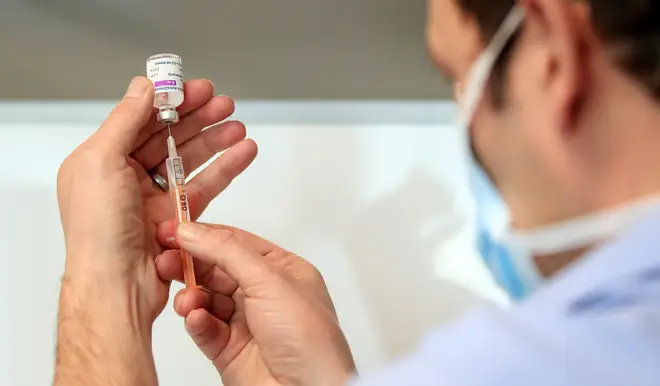
Vaccines against new coronavirus variants should be ready by October, the team behind the Oxford University/AstraZeneca jab has said.
In a media briefing hosted by AstraZeneca, Professor Andrew Pollard, director of the Oxford vaccine group, said work on designing a new vaccine could be completed rapidly.
It comes after studies have shown that variants of coronavirus with the worrying E484K mutation could make vaccines less effective, though they are still expected to offer good protection against illness and severe disease.
Read more: Don't go shopping in variant-risk areas if you don't have to, Hancock tells LBC
Prof Pollard said: "I think the actual work on designing a new vaccine is very, very quick because it's essentially just switching out the genetic sequence for the spike protein, for the updated variants.
"And then there's manufacturing to do and then a small scale study.
Matt Hancock inspired by Contagion in vaccine strategy
"So all of that can be completed in a very short period of time, and the autumn is really the timing for having new vaccines available for use rather than for having the clinical trials run."
Sir Mene Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, added: "Our ambition is to be ready for the next round of immunisations that may be necessary as we go into next winter. That's what we're aiming for."
He continued: "We're very much aiming to try and have something ready by the autumn. So, this year."
Read more: One dose of Oxford Covid jab can 'reduce transmission of coronavirus by two thirds'
Prof Pollard said it was likely that clinical trials on new vaccines for dealing with the variants of Covid-19 would involve "hundreds" of people at the most.
"That's a discussion which is ongoing with regulators about exactly what the data package is that they would need," he said.
"The reason why it's such a small number is because with an updated vaccine, the question is whether immune responses still look the same but against the new variants as they emerge.
Psychological impact of further Covid mutations: explained
"We don't need to run studies on a large scale to prove efficacy. And so that's why they're much quicker and much smaller to conduct."
Their comments came after it was revealed that the Oxford/AstraZeneca vaccine could cut Covid-19 transmission rates by 67%, in news which has been hailed by a leading pharmacologist as the "holy grail" of the global vaccine rollout.
Preliminary results of a study conducted by researchers at the University of Oxford found the efficacy from two standard doses of the vaccine administered three months apart to be 82.4%.
Before these results, little was known about how effective the Covid-19 vaccines were at preventing transmission of the disease.
The potential it could dramatically cut transmission after just one dose that will mean lockdown measures can be lifted sooner, a former chair at the Faculty of Pharmaceutical Medicine said.
Dr Gillies O'Bryan-Tear said the results, which have yet to be peer reviewed, were the first definitive estimate of the impact of vaccination on transmission rates.
"If the effect on transmission is confirmed for the Pfizer vaccine too, this would be a very positive," he said.