
Clive Bull 1am - 4am
5 December 2021, 09:53
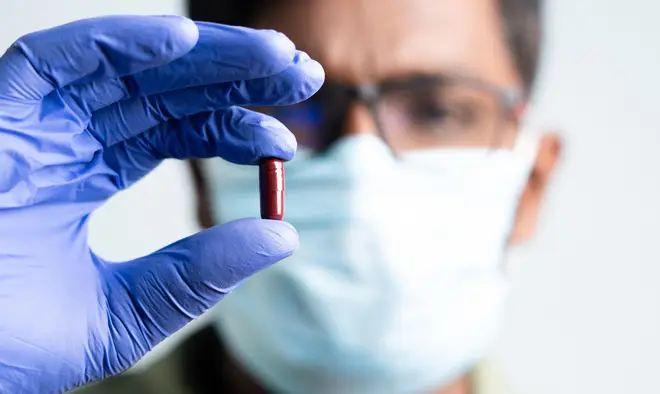
The first at-home treatment for covid could be offered to vulnerable patients before Christmas in the race to protect them from the Omicron variant.
The Molnupiravir antiviral pill - also known as Lagevrio - was approved for use last month in the UK, the first country to do so.
Health Secretary Sajid Javid described the move as a "game-changer".
The Sunday Telegraph reports the NHS is expected to deliver the treatment to clinically vulnerable and immunosuppressed patients who test positive for coronavirus within 48 hours.
Read more: Hammer blow' for travel industry as pre-departure Covid tests return for all UK arrivals
Hospitals and GPs have reportedly been informed that coronavirus medicine delivery units have been established to deliver the treatment as quickly as possible after a clinically vulnerable person contracts the virus.
It comes after England's chief medical officer Professor Chris Whitty cautioned the public over the pill, saying: "I think we probably need to do a rethink of it just to make sure with the new variant, we're targeting in the right direction."
Read more: Pre-departure Covid tests reintroduced for travellers to UK amid Omicron fears
Government reintroduces pre-departure Covid tests for entry to the UK
A Department of Health and Social Care spokesman said: "The UK has proven itself to be a world-leader in identifying and rolling out effective treatments for Covid-19, including through Government-backed national trials.
"The Government's antivirals taskforce was launched to identify treatments for UK patients who have been exposed to Covid-19 to take at home, stopping the infection spreading and speeding up recovery time.
"There are a number of exciting opportunities in the pipeline and we will provide further details in due course."