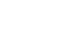
Nick Ferrari 7am - 10am
27 November 2020, 00:15 | Updated: 27 November 2020, 00:25
A coronavirus vaccine rollout in the UK could be a step closer after the regulator was formally asked by the Government to assess the Oxford University/AstraZeneca drug as soon as possible.
The move "marks a significant first step in getting the vaccine approved for deployment" if it meets the necessary standards, the Department of Health and Social Care (DHSC) said.
It comes a week after the Medicines and Healthcare products Regulatory Agency (MHRA) was formally asked by the Government to assess the Pfizer/BioNTech vaccine.
Read more: 'Don't do stupid things' over Christmas, Chief Medical Officer warns
Health secretary Matt Hancock said: "We are working tirelessly to be in the best possible position to deploy a vaccine as soon as one is approved by the independent regulator the MHRA.
"We have formally asked the regulator to assess the Oxford/AstraZeneca vaccine, to understand the data and determine whether it meets rigorous safety standards."
Hancock ordered 70 million more doses of Oxford vaccine than advised
Meanwhile, British scientists have defended Oxford University and AstraZeneca after questions were raised about the results of their vaccine trial.
AstraZeneca said it will most likely carry out a further global clinical trial to assess the efficacy of the jab after a surprise result found 90% protection was achieved when people were given a half dose followed by a full dose.
Read more: Oxford Covid vaccine: What are the side effects and how effective is it?
The pharmaceutical giant has acknowledged the finding was as a result of a dosing error, but said it did not expect any new trial to delay regulatory approval in countries including the UK.
US scientists questioned a lack of detail in the trial results published last week and the scientific head of the US's Operation Warp Speed - the programme to supply America with vaccines - told US reporters the half-dose regime was only given to people aged 55 and under.
Ethics expert addresses how Covid vaccines should be distributed
Scientists across the globe are hoping to find vaccines that work in older people - the group who are most at risk from Covid-19.
DHSC said the MHRA has already started a "rolling review" of the Oxford/AstraZeneca vaccine and that once the regulator receives the full safety, efficacy and quality data from the company, its scientists and clinicians "stand ready to progress its assessment of the vaccine".
Read more: Ethics expert addresses how Covid vaccines should be distributed
The Government said it has secured access to 100 million doses of the Oxford/AstraZeneca jab and 40 million of the Pfizer/BioNTech vaccine.
If approved, a vaccine could be rolled out from December, Mr Hancock has said.