
Clive Bull 1am - 4am
7 April 2021, 15:32 | Updated: 7 April 2021, 19:20

Under-30s to be offered alternative to Oxford/AstraZeneca jab
People in the UK under the age of 30 will be offered an alternative to the Oxford-AstraZeneca jab, after the UK medicines regulator found a possible link between the specific vaccine and a rare blood clot condition.
Those between 18 and 29, who do not have an underlying health condition, will be offered the Pfizer-BioNTech vaccine or the Moderna jab in preference to the AstraZeneca jab, where it is available.
People who are over 29 or have had the jab already should still receive their first and second AstraZeneca jab when offered.
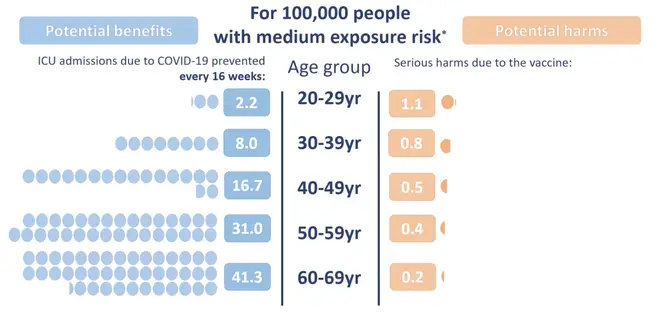
The move is based on an assessment that the risk-benefit for those who are under 30-years-old is lower, as they are at a lower risk of becoming seriously ill from Covid-19.
However, the regulator stressed that they are acting on an "abundance of caution", with the risk of a blood clot "extremely small.
Read more: LIVE: UK regulator to hold news conference at 3pm on Oxford/AstraZeneca Covid-19 jab
Read more: AstraZeneca vaccine trial on children paused amid blood clot fears

PM does not believe jab 'course correction' will affect roadmap
The move follows a safety review from the UK's Medicines and Healthcare products Regulatory Agency, which looked at concerns that the Oxford-AstraZeneca vaccine may be linked to "very rare and specific types of blood clots with low platelets".
Speaking at a press conference, Dr June Raine, Chief Executive of the MHRA, said the evidence is "firming up" on the potential side effect but that more evidence is needed to establish "beyond doubt" that there is a link.
She added that the "benefits continue to outweigh the risks for the vast majority of people".
According to their data around four in a million people who have received the vaccine developed blood clots, based on 20.2 million people receiving the jab by 31 March 2021.
"We are not advising a stop to any vaccination for any individual in any age group," Professor Wei Shen, chair of the Joint Committee on Vaccination and Immunisation said.
"We are advising a preference for one vaccine over another vaccine for a particular age group, out of the upmost caution, rather than because we have any serious safety concerns."
Meanwhile, Sir Munir Pirmohamed, Chair of the Committee of Human Medicines, also stressed that the risk of blood clots in those who catch Covid is much higher than those who take the vaccine.
Read more: Former head of UK vaccines regulator: 'No reservations' about AstraZeneca jab
Blood clots from AZ jab 'such a rare' reaction, says former MHRA chief
As the MHRA continue to investigate the cases, as a precautionary measure, anyone who has symptoms four days or more after vaccination is advised to seek prompt medical advice, such as:
Aspirin is 'probably more dangerous' than Oxford jab says SAGE member
Professor Jonathan Van-Tam, Deputy Chief Medical Officer for England described the move as a "course correction" and stressed the potential side effects are "vanishingly rare".
"This is a massive beast that we are driving along at enormous pace, with enormous success, this vaccine programme.
"If you sail a massive liner across the Atlantic then it is not really reasonable that you aren't going to have to make at least one course correction during that voyage."
No safety issues have been raised with either the Pfizer-BioNTech or the Moderna jabs, which operate with a different technology.
Read more: First dose of Moderna vaccine given in UK as carer Elle Taylor gets jab

First dose of Moderna vaccine given in UK
Prof Van-Tam said he has spoken with the NHS and due to the supply of alternative vaccines there should be a "zero or negligible" impact on the UK's vaccine rollout.
"That of course is contingent on us getting the supplies that we expect to get of the alternative vaccines, which are the Pfizer vaccine, currently in use, and the Moderna vaccine that we hope to bring into deployment from mid April in England."
He continued: "The NHS has a message: that we will get the right vaccine to you, in the right time, according to the new JCVI advice.
"There might be a small delay sometimes, there might be a slightly greater distance that some people are asked to travel, but the NHS is all over this."
Read more: First UK doses of Moderna vaccine to be administered today in Wales

The benefits of the AstraZeneca vaccine outweigh the risks
The European Medicines Agency have also announced a similar review, concluding that the "unusual blood clots with low blood platelets should be listed as very rare side effects" of the AstraZeneca vaccine.
However, they added "the chance of having this occur is very low" and "the benefits of the vaccine continue to outweigh the risks for people who receive it".
Read more: Blood clots should be listed as a 'very rare' side effect of AstraZeneca jab: EU regulator

'Unusual blood clotting should be listed as a possible side effect of AstraZeneca vaccine'
On Tuesday, during a visit to an AstraZeneca factory Prime Minister Boris Johnson urged people to trust the MHRA to regulate safely and urged people to get their jab.
"On the Oxford/AstraZeneca vaccine, the best thing people should do is look at what the MHRA say, our independent regulator - that's why we have them, that's why they are independent," he told reporters.
"Their advice to people is to keep going out there, get your jab, get your second jab."
AstraZeneca said: "Neither agency identified any risk factors, such as age or gender, or a definite cause for these extremely rare events.
"However, they came to the view that these events have a possible link to the vaccine and requested they be listed as an extremely rare potential side effect.
"Overall, both of these reviews reaffirmed the vaccine offers a high-level of protection against all severities of COVID-19 and that these benefits continue to far outweigh the risks.
"AstraZeneca has been actively collaborating with the regulators to implement these changes to the product information and is already working to understand the individual cases, epidemiology and possible mechanisms that could explain these extremely rare events."
The World Health Organisation said a blood clots link was plausible but unconfirmed.