
Clive Bull 1am - 4am
31 March 2021, 12:37 | Updated: 31 March 2021, 13:26
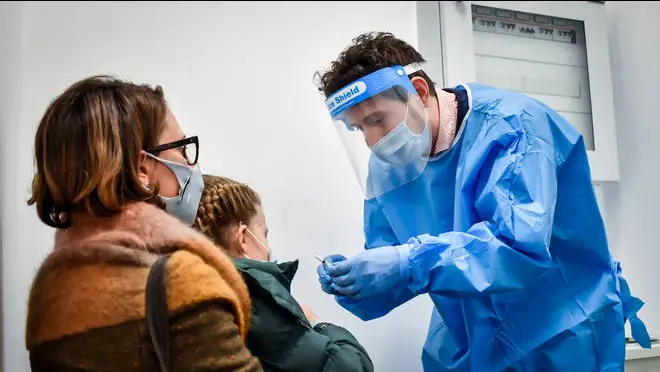
Pfizer has announced its Covid-19 jab is safe and highly effective among children as young as 12, in a major step towards the possibility of giving secondary school students the vaccine.
In an American study of 2,260 volunteers aged 12 to 15, preliminary data showed no cases of Covid-19 among fully vaccinated teens, compared with 18 among those given dummy shots, Pfizer reported.
Although the small study has not yet been published, researchers also promisingly reported that they saw higher levels of virus fighting antibodies amongst the 12 to 15-year-olds than have been seen in young adults.
Read more: Covid vaccine for children: Will kids get a Covid-19 immunisation?
Read more: Final day of shielding for almost 4 million people in England and Wales
Children had side effects similar to young adults, the company said, mainly pain, fever, chills and fatigue, particularly after the second dose.
The study will continue to track participants for two years for more information about long-term protection and safety.

Boris Johnson: Government must repay a generation of children
Currently in the UK only those aged 18 and older are able to get a Covid vaccine, as previously there had not been enough data from trials in the age group.
Reports suggest children in the UK could be given their first jabs as early as August under provisional government plans.
A new trial was also confirmed earlier this year to test how well the Oxford-AstraZeneca jab works in children.
Read more: What are the approved coronavirus vaccines in the UK?
Read more: Digital vaccine certificates for European travel 'ready in June at latest'
Around 300 volunteers will have the first dose with researchers evaluating their immune response to the vaccination.
The trial will be using children aged six to 17 and volunteers who live near the four study sites such as the University of Oxford are being asked to step forward.
Results also are expected soon from a US study of Moderna's vaccine in 12 to 17-year-olds, with jabs arriving in the UK in April.
Mother in her 70s seeks advice as her son won't get vaccine
Pfizer and BioNTech say they plan to ask the US Food and Drug Administration and European regulators to allow emergency use of the vaccine starting at the age of 12.
"We share the urgency to expand the use of our vaccine," Pfizer chief executive Albert Bourla said in a statement. He expressed "the hope of starting to vaccinate this age group before the start of the next school year" in the US.
But in a sign that the findings were promising, the FDA has already allowed both companies to begin US studies in children aged 11 and younger, working their way to as young as six months.